Dicerna Pharmaceuticals Inc Pipeline / Dicerna Announces Two Targets Meet Preclinical Proof Of Principle Criteria In Neurodegeneration And Pain Under Global Research Collaboration And Licensing Agreement With Lilly Business Wire
Addressing AAT deficiency-associated liver disease AATLD Belcesiran is our GalXC product candidate for the treatment of alpha-1 antitrypsin AAT deficiency-associated liver disease AATLD. Dicerna made the strategic decision to develop its products through one of two methods by either pooling resources and expertise with other pharmaceutical.
Dicerna Reasonably Priced Play In Rnai Space Nasdaq Drna Seeking Alpha
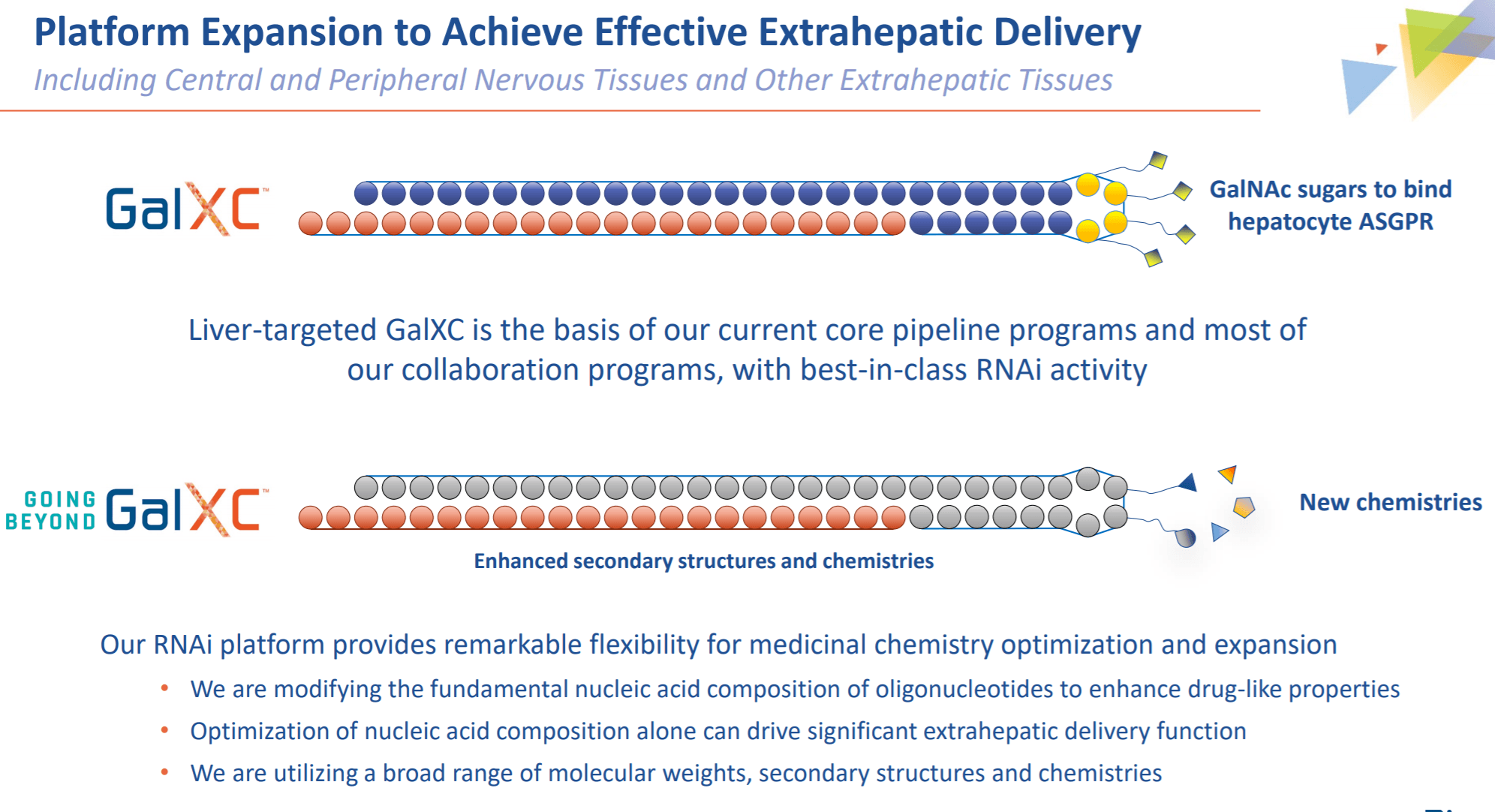
Our mission is to discover and develop potent selective and safe RNAi therapies to treat diseases including cardiometabolic viral chronic liver and complement-mediated diseases as well as neurodegenerative diseases and pain.
Dicerna pharmaceuticals inc pipeline. DRNA is a biopharmaceutical company focused on discovering developing and commercializing medicines that are designed to leverage ribonucleic acid interference RNAi to silence selectively genes that cause or contribute to disease. Dicernas Pipeline and Strategy. The potential to treat HBV.
DRNA is a biopharmaceutical company focused on discovering developing and commercializing medicines that are designed to leverage ribonucleic acid interference RNAi to silence selectively genes that cause or contribute to disease. View the latest DRNA company infomation and executive bios. The Company sets high standards for the Companys employees officers and directors.
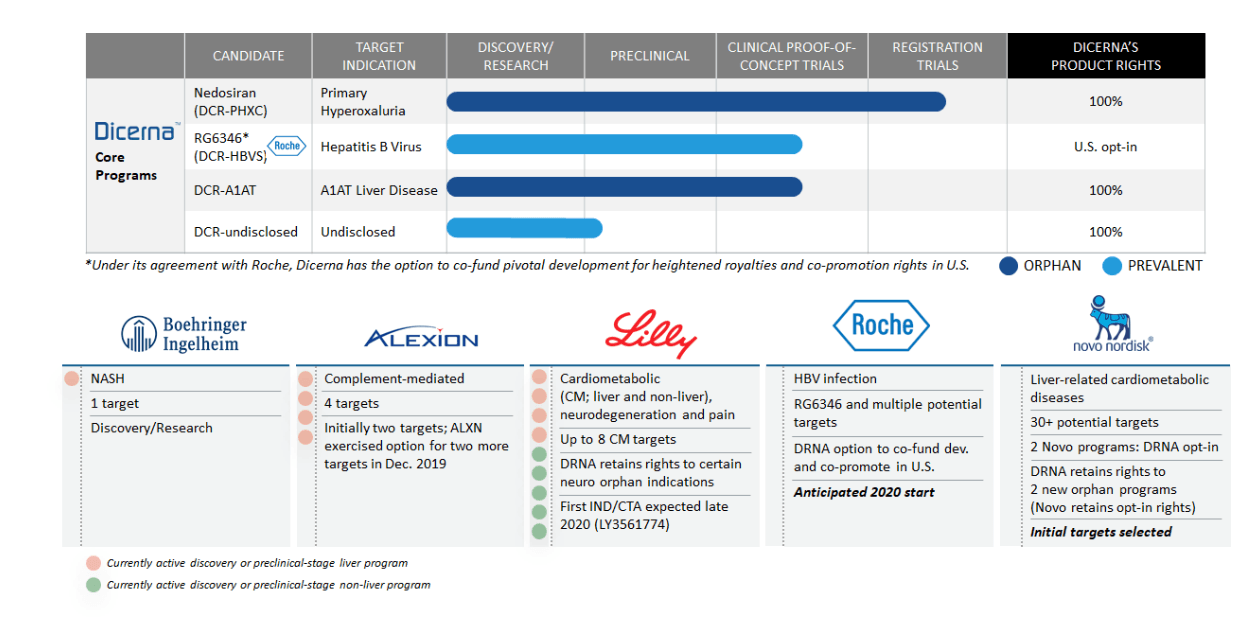
Between Dicerna and our collaborative. Novo Nordisks juicy buyout offer values Dicerna Pharmaceuticals at 33 billion. In addition to our own pipeline of core discovery and clinical candidates Dicerna has established collaborative relationships with some of the worlds leading pharmaceutical companies including Novo Nordisk AS Roche Eli Lilly and Company Alexion Pharmaceuticals Inc Boehringer Ingelheim International GmbH and Alnylam Pharmaceuticals Inc.
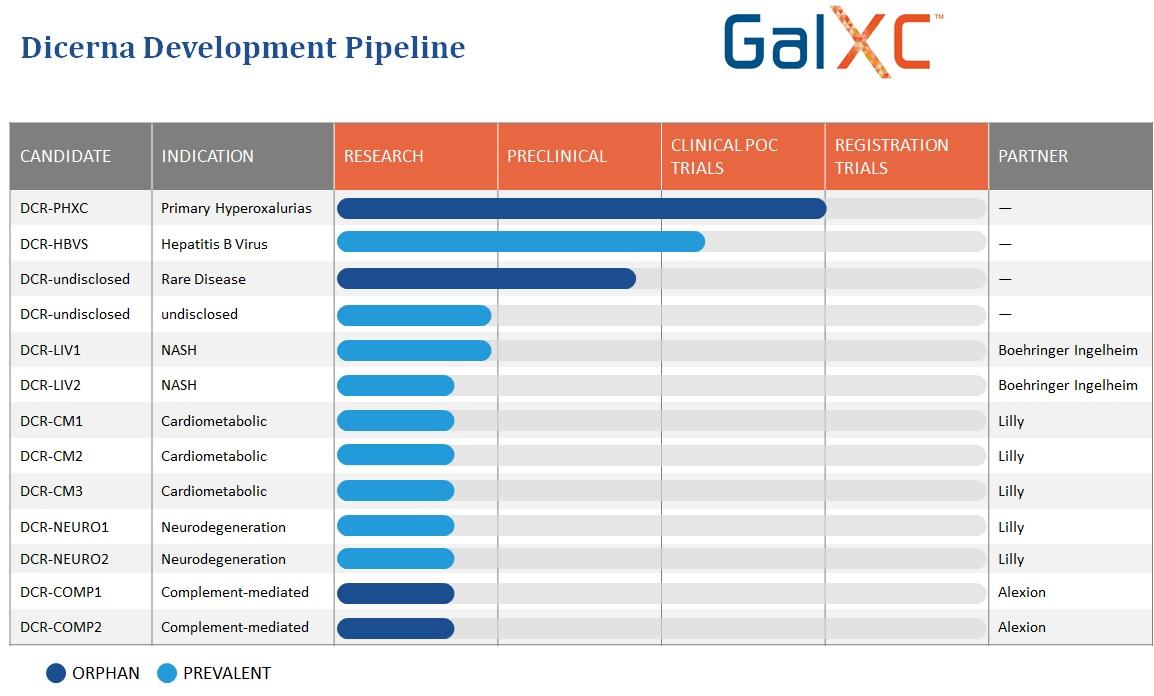
View DRNA revenue estimates and earnings estimates as well as in-depth analyst breakdowns. At Dicerna we are evaluating nedosiran for the treatment of all three known types of PH as part of the PHYOX clinical program RG6346 in collaboration with Roche is in development for the treatment of chronic hepatitis B virus HBV infection and belcesiran formerly DCR-A1AT is in development for the treatment of alpha-1 antitrypsin AAT deficiency-associated liver disease AATLD as. Dicernas lead investigational RNAi therapy in development for the treatment of PH.
DRNA is a clinical stage pharmaceutical company focused on developing new medicines and treatments for a wide range of diseases using RNA interference RNAi technology. Using our proprietary GalXC and GalXC-Plus RNAi technologies Dicerna is. It is the duty of the Board of Directors to serve as a prudent fiduciary for shareholders and to oversee the management of the Companys business.
As well as over 20 discovery research stage programs in multiple tissues or cell types and looking across this entire pipeline of. Between Dicerna and our. NasdaqDRNA a leading developer of investigational ribonucleic acid interference RNAi therapeutics today announced that the company has resolved all litigation with Alnylam Pharmaceuticals Inc.
PH is a family of ultra-rare life-threatening genetic disorders that initially manifest with complications in the kidneys. RG6346 is an investigational GalXC RNAi therapy in development with Roche for the treatment of chronic hepatitis B virus HBV infection Chronic HBV increases the risk of developing liver failure liver cancer or cirrhosis a condition that permanently scars the liver. In addition to our own pipeline of core discovery and clinical candidates Dicerna has established collaborative relationships with some of the worlds leading pharmaceutical companies including Novo Nordisk AS Roche Eli Lilly and Company Alexion Pharmaceuticals Inc Boehringer Ingelheim International GmbH and Alnylam Pharmaceuticals Inc.
Research and ratings by Barrons. 20 2018-- Dicerna Pharmaceuticals Inc. About Dicerna Pharmaceuticals Inc.
Company and executive profile by Barrons. And previously served as corporate vice president of global branded products at Teva Pharmaceutical Industries Limited after Teva acquired Cephalon in October 2011. Dicerna Pharmaceuticals Inc.
Using our proprietary GalXC and GalXC-Plus RNAi technologies Dicerna is committed to. Implicit in this philosophy is the importance of sound corporate governance. RG6346 has the potential to contribute to the ultimate goal.
Dicerna Pharmaceuticals Inc. DRNA is a biopharmaceutical company focused on discovering developing and commercializing medicines that are. In addition to our own pipeline of core discovery and clinical candidates Dicerna has established collaborative relationships with some of the worlds leading pharmaceutical companies including Novo Nordisk AS Roche Eli Lilly and Company Alexion Pharmaceuticals Inc Boehringer Ingelheim International GmbH and Alnylam Pharmaceuticals Inc.
The Investor Relations website contains information about Dicerna Pharmaceuticalss business for stockholders potential investors and financial analysts. Buchi is the former President and Chief Executive Officer of Cephalon Inc. About Dicerna Pharmaceuticals Inc.
Dicerna Pharmaceuticals Inc NASDAQDRNA Q3 2021 Earnings Call Nov 9 2021 830 am. Companys Resources Focused on Advancement of All Key Pipeline Programs. Dicerna Pharmaceuticals Inc is a biopharmaceutical company using ribonucleic acid interference RNAi to develop medicines that silence genes that cause disease.
The Board of Directors of Dicerna Pharmaceuticals Inc. We are developing nedosiran our most advanced RNAi drug candidate utilizing our proprietary GalXC RNAi technology for the treatment of primary hyperoxaluria PH. At the moment Dicernas pipeline has a handful of midstage candidates and one.
Between Dicerna and our collaborative. Alpha-1 antitrypsin deficiency AATD is a rare inherited disorder that can lead to liver disease in children and liver and lung disease in adults. Kevin Buchi joined the Dicerna board of directors in August 2018 and was appointed Chairman of the Board in January 2019.
Compassionate use policy Dicerna Pharmaceuticals Inc. To fulfill its. Dicerna Pharmaceuticals Inc.
Diving Into Dicerna Pharmaceuticals Nasdaq Drna Seeking Alpha
Dicerna Pharmaceuticals Continues To Successfully Hit The Target Nasdaq Drna Seeking Alpha

Dicerna To Join Nasdaq Biotechnology Index Business Wire

Dicernacorporatepresenta Dicerna Pharmaceuticals Inc 2020 Current Report 8 K

Dicerna To Report Third Quarter 2021 Financial Results On Nov 9 2021 Swasth Samachar

Information About Dicerna S Pipeline Dicerna Pharmaceuticals

Alnylam And Dicerna Form Rnai Therapeutics Collaboration On Alpha 1 Antitrypsin Deficiency Associated Liver Disease And Complete Cross License Agreement For Primary Hyperoxaluria Programs Business Wire

Dicerna Pharmaceuticals Small Cap Nation Event Solebury Trout Access

Novo Nordisk Ubernimmt Dicerna Pharmaceuticals Aktienkurs Steigt Um 80 Invezz

Rnai Developer Dicerna Pharmaceuticals To Ipo Nanalyze
Dicerna Pharmaceuticals Inc Aktie

Dicerna Announces Two Targets Meet Preclinical Proof Of Principle Criteria In Neurodegeneration And Pain Under Global Research Collaboration And Licensing Agreement With Lilly Business Wire
Information About Dicerna S Pipeline Dicerna Pharmaceuticals

Dicerna Pharmaceuticals Drug Developments Pipeline Prospector

Dicerna Announces Second Quarter 2021 Financial Results And Provides A Business Update Citybiz

Dicerna Raises 70m Reels In Losses For Q4 Drug Delivery Business

